Flourish Research - Chicago (Ravenswood)
Psoriatic Arthritis Study Self-Scheduler
Basic Criteria:
- Be 18 years or older
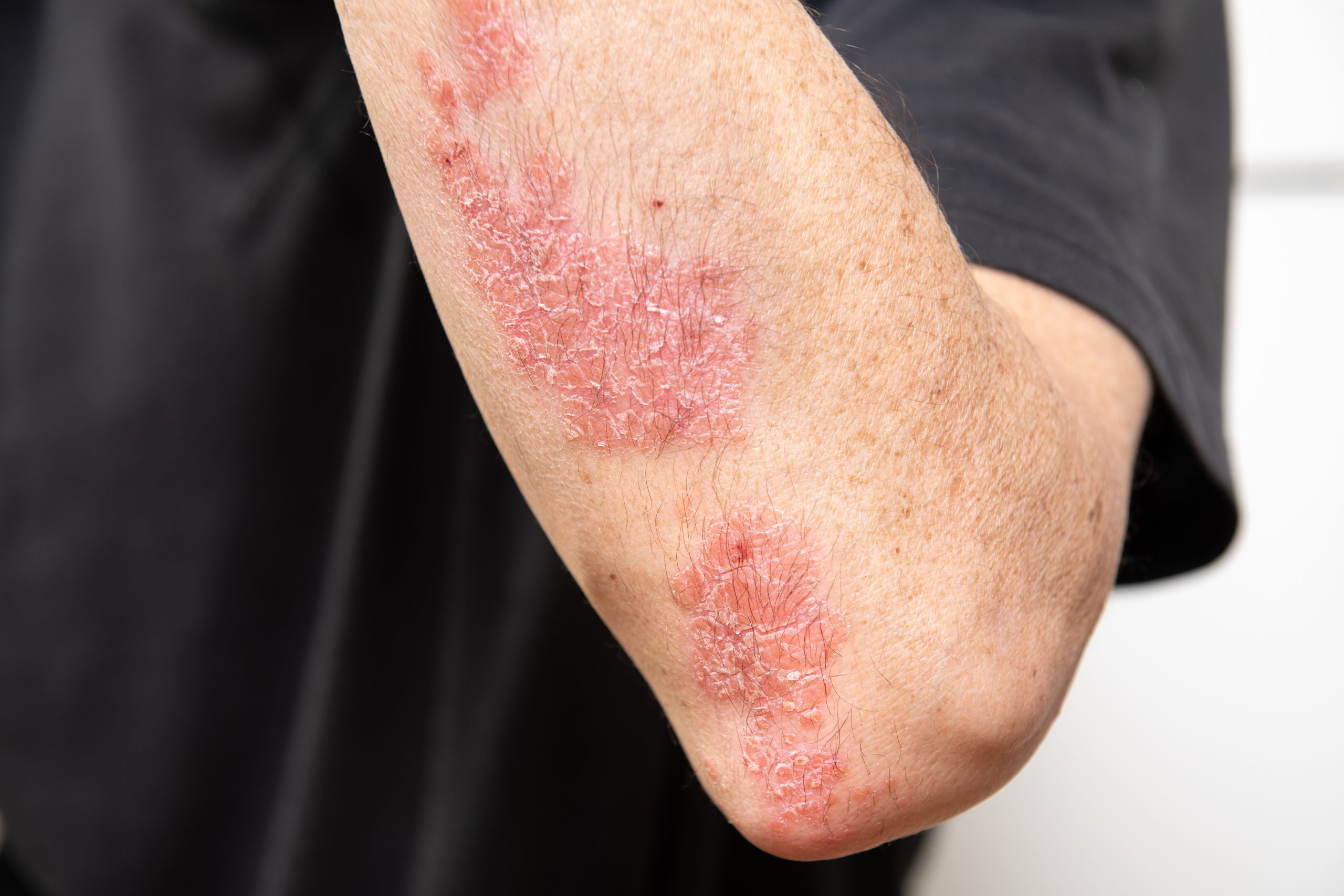
- Be diagnosed with Active Psoriatic Arthritis
- Have a BMI of 30+ OR a BMI between 27 and 30 with at least one of the following conditions: Hypertension, Dyslipidemia, Type 2 Diabetes, Obstructive Sleep Apnea, or Cardiovascular Disease
- Be treated with ixekizumab for the last 2-4 months
Please note that additional study criteria will apply.
Answers Must Be No:
- If female of childbearing potential, are you pregnant, breastfeeding, or planning on becoming pregnant?
- Do you have type 1 diabetes?
- Have you been receiving Ixekizumab for more than 4 months?
- Do you have a personal or family history of medullary thyroid cancer?
- Do you have multiple endocrine neoplasia type 2?
- Have you ever had a history of chronic or acute pancreatitis?
- Do you have a history of proliferative diabetic retinopathy, diabetic maculopathy, or nonproliferative diabetic retinopathy that requires acute treatment?
- Have you experienced severe hypoglycemia or hypoglycemia in the last 6 months?
- Do you have a history of severe gastrointestinal complications, such as gastroparesis, gastroesophageal reflux disease, dyspepsia, or chronic nausea, constipation, or vomiting?
- Do you have a history of liver disease or high liver enzymes?
- Have you ever had a history of suicidal ideation or behavior?
- Have you experienced kidney disease in the last 6 months?
- Do you have end-stage osteoarthritis of the hip or knee?
- Do you have HIV, Hepatitis B, or Hepatitis C?
Answers Must Be Yes:
- Are you 18 years or older?
- Do you have a BMI of 30+, OR do you have a BMI between 27 and 30 with at least one of the following conditions: Hypertension, Dyslipidemia, Type 2 Diabetes, Obstructive Sleep Apnea, or Cardiovascular Disease?
- Have you been diagnosed with Active Psoriatic Arthritis?
- Are you willing/able to start taking tirzepatide within a month of the study beginning?
PLEASE NOTE: Please confirm your eligibility before your appointment by calling 312-931-8883
- If you do not see an available appointment, please call 312-931-8883 for availability.
- Transportation may be available. Call 312-931-8883. Compensation may be available for time and travel.
By signing up, you agree to receive text messages and emails about this and similar studies near you. You can unsubscribe at any time. Text messages and data rates may apply.
Schedule Appointment
Flourish Research News
Stay current with the latest clinical trial news and learn how Flourish Research is supporting the advancement of life-changing therapeutics.